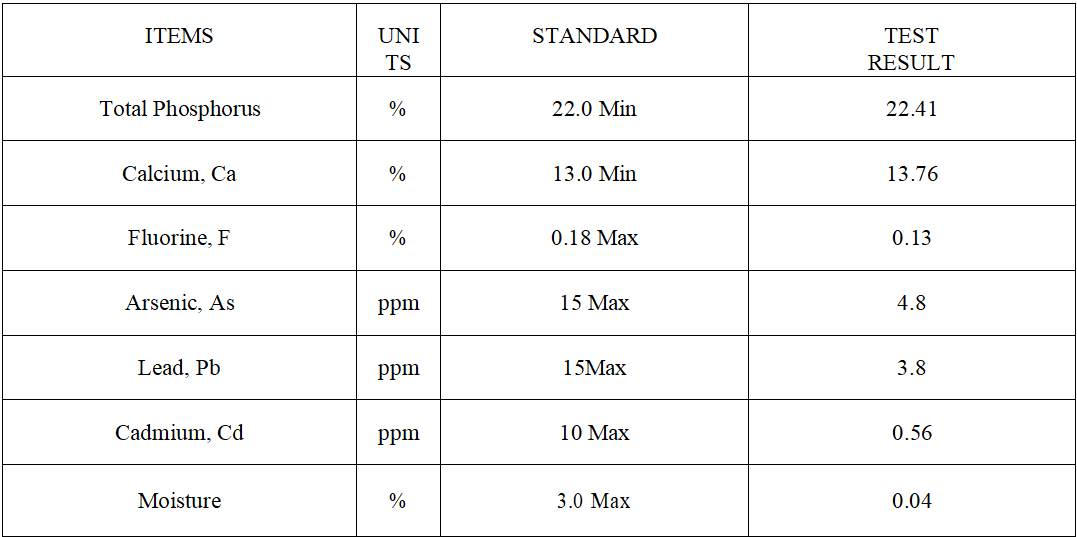
Monocalcium Phosphate Feed Additive 22 – 23%
Product Description:
Monocalcium phosphate (MCP) is a chemical compound with the formula Ca(H2PO4)2. It is commonly used as a leavening agent in baking powder and as a nutritional supplement in animal feed. Here are some key points about monocalcium phosphate:
Chemical Structure: Monocalcium phosphate is a calcium acid salt of phosphoric acid. It consists of calcium ions (Ca2+) and dihydrogen phosphate ions (H2PO4-) in a 1:2 ratio.
Production: Monocalcium phosphate is typically produced by reacting calcium carbonate (limestone) with phosphoric acid. The reaction forms monocalcium phosphate along with calcium sulfate (gypsum), which is insoluble and can be removed by filtration.
Properties:Appearance: Monocalcium phosphate is usually found as a white powder or granular solid.
Solubility: It is soluble in water, with the solubility increasing as the temperature rises.
pH: Monocalcium phosphate is acidic in nature, contributing to its use as a leavening agent in baking.
Uses:
Baking: Monocalcium phosphate is commonly used in baking powder formulations as a leavening agent. When combined with baking soda (sodium bicarbonate) and an acid, such as cream of tartar, it produces carbon dioxide gas, which causes dough or batter to rise and become light and fluffy during baking.
Animal Feed: Monocalcium phosphate is used as a source of calcium and phosphorus in animal feed formulations. It provides essential minerals necessary for bone development, muscle function, and overall animal health. In addition to its nutritional benefits, it can also improve the palatability and texture of feed pellets.
Safety Considerations: Monocalcium phosphate is generally regarded as safe (GRAS) for use in food and animal feed by regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). However, excessive intake of phosphorus can lead to health issues in humans and animals, so it is important to use monocalcium phosphate and other phosphorus sources judiciously in formulations.